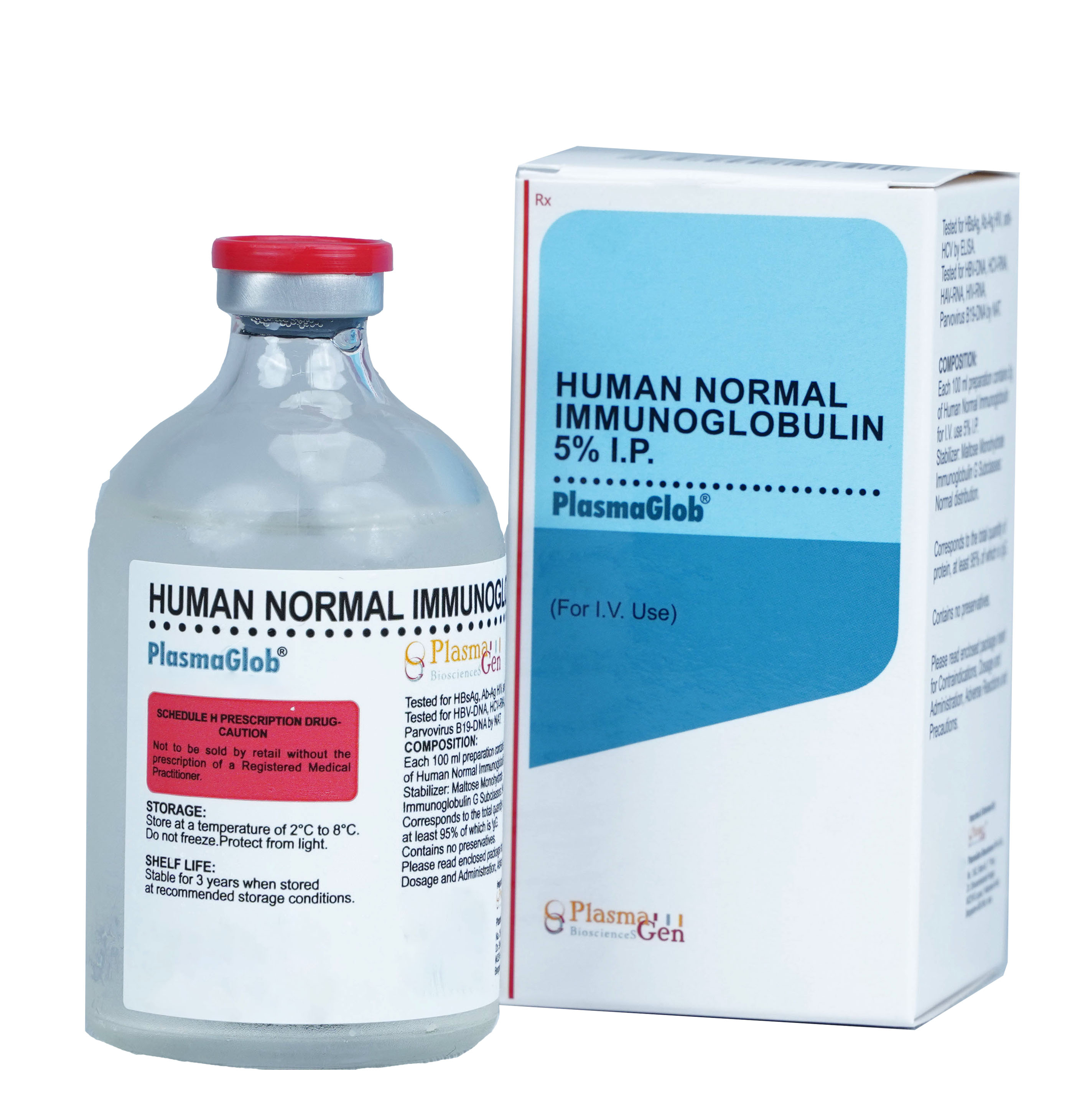
Product Description
PlasmaGlob is Normal Human Immunoglobulin for Intravenous use prepared from Indian Human Plasma from healthy voluntary donors, by Cohn Fractionation/ Chromatography process. Each unit of plasma is tested for all mandatory serological markers prescribed by National Regulatory Authority (NRA).
To increase safety profile of PlasmaGlob , the plasma used in its manufacturing and final product undergoes extensive testing from various protocols HBsAg, Ab-Ag HIV, anti-HCV by ELISA and for HBV-DNA, HCV-DNA, HAV-RNA, HIV-RNA, Parvovirus B19-DNA by NAT/PCR.
Only the non-reactive plasma units were used for manufacturing of PlasmaGlob. To further improve the margin of safety effective viral inactivation steps like solvent detergent treatment have been integrated into the manufacturing and formulation process. PlasmaGlob has undergone two steps of viral inactivation. As per the regulatory norms and Hemovigilance program, the record of plasma used in manufacturing of PlasmaGlob has been maintained for 10 years.
Indications
- Primary Immune Deficiency Diseases (PIDDs)
- Idiopathic Thrombocytopenic Purpura(ITP)
- Kawasaki Disease(KD)
- Guillain-Barré Syndrome(GBS)
- Chronic Lymphocytic Leukemia (CLL)
- Chronic Inflammatory Demyelinating Polyneuropathy (CIDP)
- Bone Marrow Transplantation (BMT) – for prevention of graft vs host disease
Storage
- Store at 2 to 8°C
- Protect from light
- Do not freeze
Shelf-life
36 months from the date of manufacture